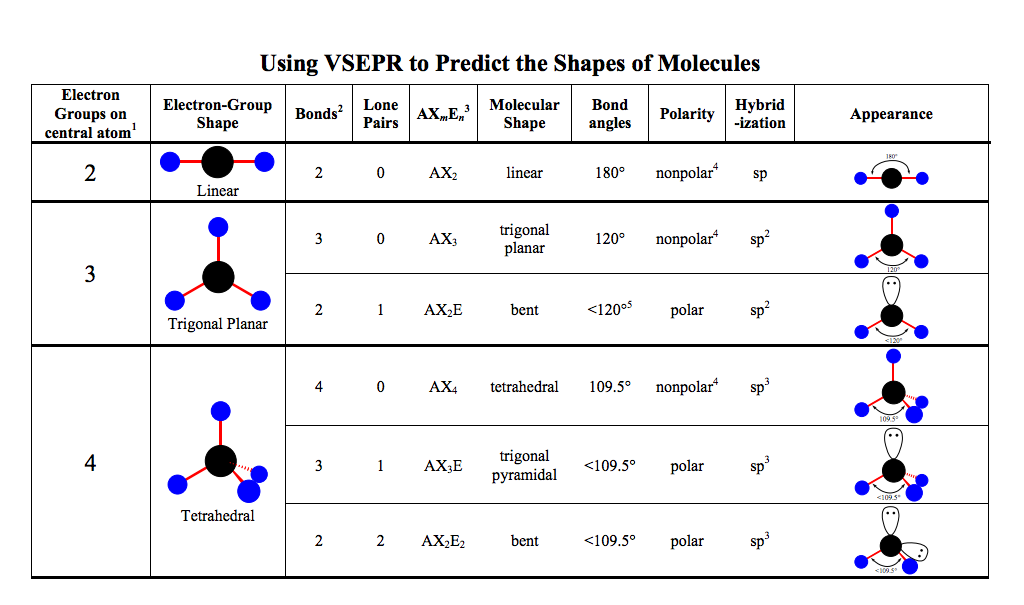
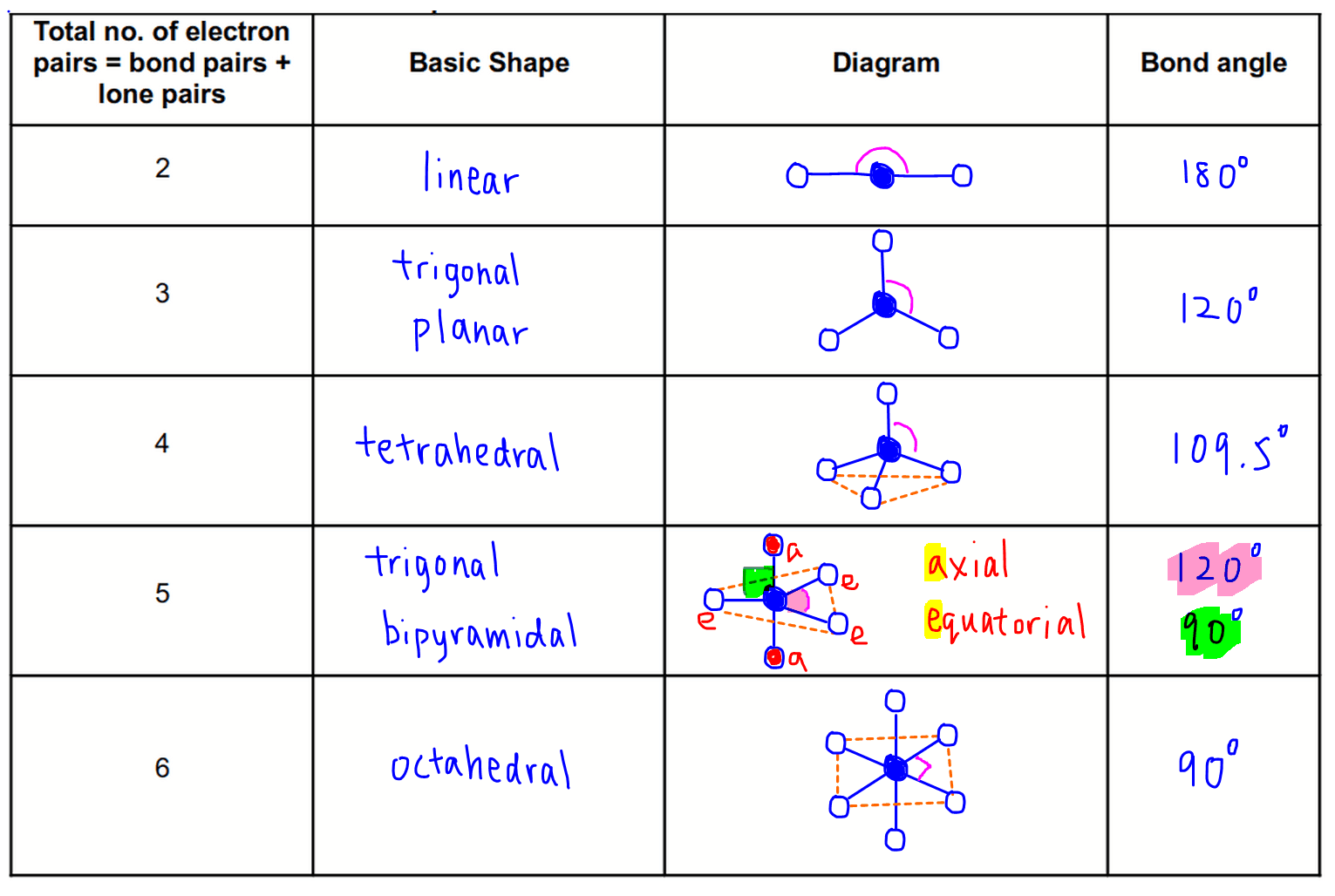
Vsepr Chart With Polarity
Vsepr Chart With Polarity - Get help with your vsepr theory homework. Understand the chart and model and enhance your knowledge with an optional quiz for practice. Vsepr stands for valence shell electron pair repulsion and is a theory for predicting the 3d shape of molecules. It involves the consideration of electron pairs that surrounds the constituent atoms. Also, see the vsepr chart. The shapes are predicted based on the repulsion of. Access the answers to hundreds of vsepr theory questions that are explained in a way that's easy for you to understand. Vsper has made the prediction of various molecule's shape very easy in the field of chemistry. Vsepr theory is used for predicting the information about the geometry of molecules depending upon how many lone pairs and bond pairs are there. The shape of a molecule can be determined by the valence shell electron pair repulsion (vsepr) theory which states that electron groups around a central atom are. Vsepr theory valence shell electron pair repulsion theory shortened as vsepr theory. The shapes are predicted based on the repulsion of. The statement of the vsepr theory is, the geometry of terminal atoms or a group of atoms. Also, see the vsepr chart. Vsepr theory is used for predicting the information about the geometry of molecules depending upon how many lone pairs and bond pairs are there. According to this theory, we can. Get help with your vsepr theory homework. Learn about vsepr theory with our engaging video lesson! The shape of a molecule can be determined by the valence shell electron pair repulsion (vsepr) theory which states that electron groups around a central atom are. Also, see the vsepr chart. Also, see the vsepr chart. Vsepr stands for valence shell electron pair repulsion and is a theory for predicting the 3d shape of molecules. Learn the postulates of vsepr theory and the application of vsepr theory in predicting the shapes of molecules. Vsepr theory is used for predicting the information about the geometry of molecules depending upon how many lone. The shapes are predicted based on the repulsion of. Vsepr theory valence shell electron pair repulsion theory shortened as vsepr theory. Get help with your vsepr theory homework. Also, see the vsepr chart. Learn the postulates of vsepr theory and the application of vsepr theory in predicting the shapes of molecules. Also, see the vsepr chart. Vsepr stands for valence shell electron pair repulsion and is a theory for predicting the 3d shape of molecules. Learn the postulates of vsepr theory and the application of vsepr theory in predicting the shapes of molecules. The statement of the vsepr theory is, the geometry of terminal atoms or a group of atoms. Vsepr. Access the answers to hundreds of vsepr theory questions that are explained in a way that's easy for you to understand. Vsepr theory is used for predicting the information about the geometry of molecules depending upon how many lone pairs and bond pairs are there. According to this theory, we can. The shapes are predicted based on the repulsion of.. It involves the consideration of electron pairs that surrounds the constituent atoms. Vsepr theory is used for predicting the information about the geometry of molecules depending upon how many lone pairs and bond pairs are there. Get help with your vsepr theory homework. Access the answers to hundreds of vsepr theory questions that are explained in a way that's easy. The shape of a molecule can be determined by the valence shell electron pair repulsion (vsepr) theory which states that electron groups around a central atom are. Also, see the vsepr chart. The shapes are predicted based on the repulsion of. Learn the postulates of vsepr theory and the application of vsepr theory in predicting the shapes of molecules. According. Also, see the vsepr chart. Get help with your vsepr theory homework. Also, see the vsepr chart. Access the answers to hundreds of vsepr theory questions that are explained in a way that's easy for you to understand. Vsepr theory valence shell electron pair repulsion theory shortened as vsepr theory. Access the answers to hundreds of vsepr theory questions that are explained in a way that's easy for you to understand. Vsper has made the prediction of various molecule's shape very easy in the field of chemistry. It involves the consideration of electron pairs that surrounds the constituent atoms. Learn about vsepr theory with our engaging video lesson! Vsepr theory. Vsepr theory valence shell electron pair repulsion theory shortened as vsepr theory. Vsper has made the prediction of various molecule's shape very easy in the field of chemistry. Access the answers to hundreds of vsepr theory questions that are explained in a way that's easy for you to understand. The shapes are predicted based on the repulsion of. Vsepr theory. Vsepr theory valence shell electron pair repulsion theory shortened as vsepr theory. The statement of the vsepr theory is, the geometry of terminal atoms or a group of atoms. Learn the postulates of vsepr theory and the application of vsepr theory in predicting the shapes of molecules. Understand the chart and model and enhance your knowledge with an optional quiz. Understand the chart and model and enhance your knowledge with an optional quiz for practice. Also, see the vsepr chart. It involves the consideration of electron pairs that surrounds the constituent atoms. Vsepr theory valence shell electron pair repulsion theory shortened as vsepr theory. Learn the postulates of vsepr theory and the application of vsepr theory in predicting the shapes of molecules. Learn about vsepr theory with our engaging video lesson! Vsper has made the prediction of various molecule's shape very easy in the field of chemistry. The shape of a molecule can be determined by the valence shell electron pair repulsion (vsepr) theory which states that electron groups around a central atom are. Vsepr stands for valence shell electron pair repulsion and is a theory for predicting the 3d shape of molecules. According to this theory, we can. The statement of the vsepr theory is, the geometry of terminal atoms or a group of atoms. Get help with your vsepr theory homework. Also, see the vsepr chart.Printable Vsepr Chart
6.9 VSEPR and Polarity Chemistry LibreTexts
Printable Vsepr Chart
VSEPR
Vsepr Model Chart A Visual Reference of Charts Chart Master
VSEPR
[DIAGRAM] Hcl Vsepr Diagram
Vsepr model molecular geometry calculator electronicgnom
Vsepr
VSEPR Chart Valence Shell Electron Pair Repulsion Theory
The Shapes Are Predicted Based On The Repulsion Of.
Learn The Postulates Of Vsepr Theory And The Application Of Vsepr Theory In Predicting The Shapes Of Molecules.
Vsepr Theory Is Used For Predicting The Information About The Geometry Of Molecules Depending Upon How Many Lone Pairs And Bond Pairs Are There.
Access The Answers To Hundreds Of Vsepr Theory Questions That Are Explained In A Way That's Easy For You To Understand.
Related Post:




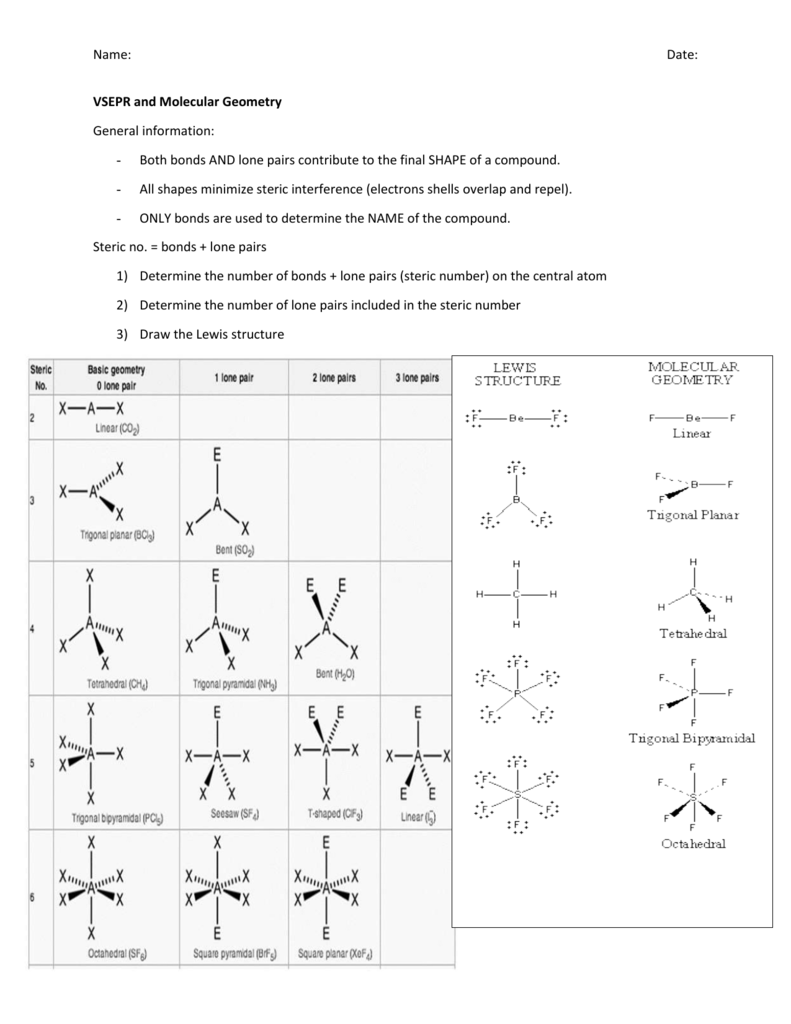

![[DIAGRAM] Hcl Vsepr Diagram](https://i.ytimg.com/vi/40mG2rQlLpk/maxresdefault.jpg)