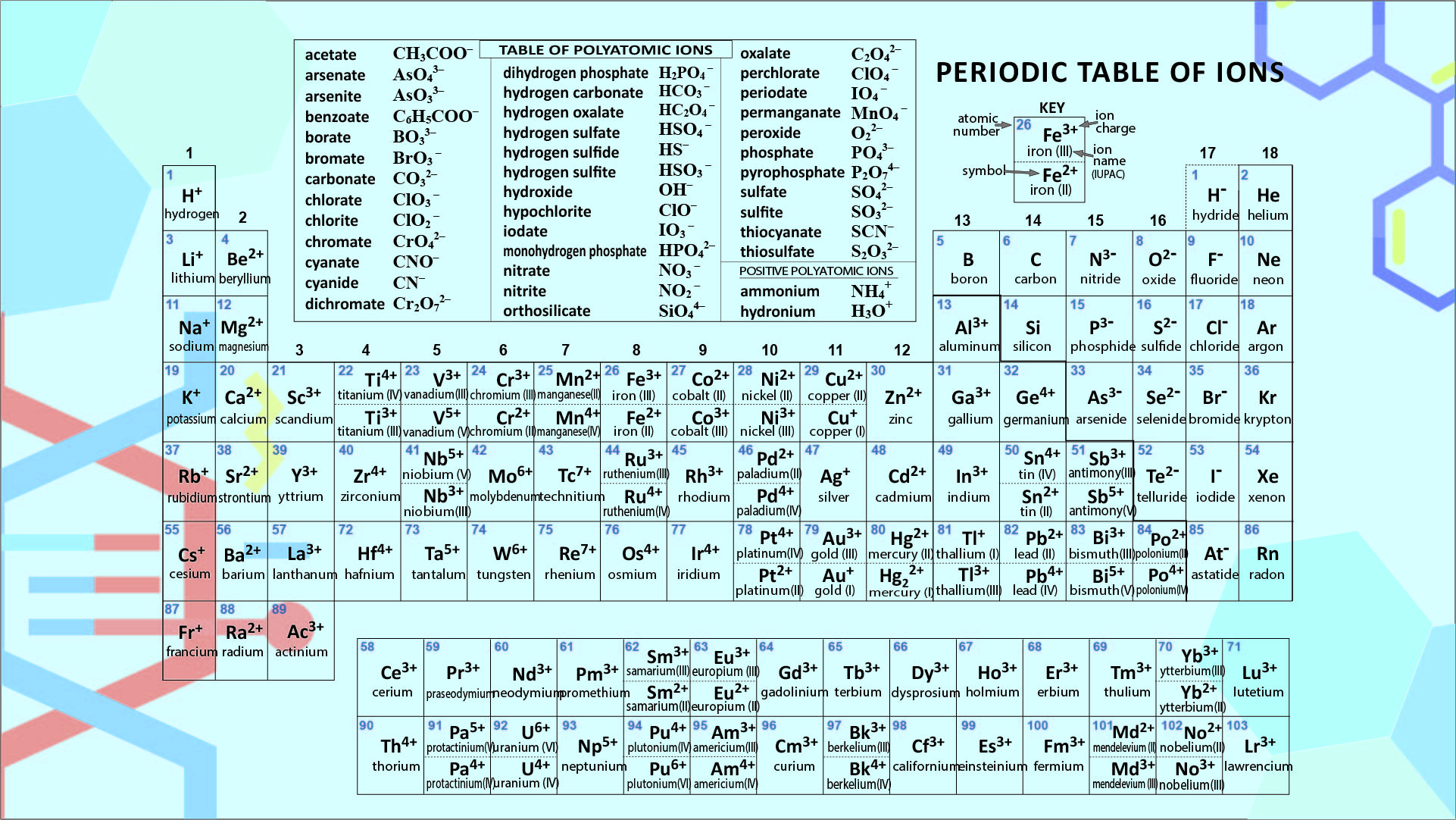
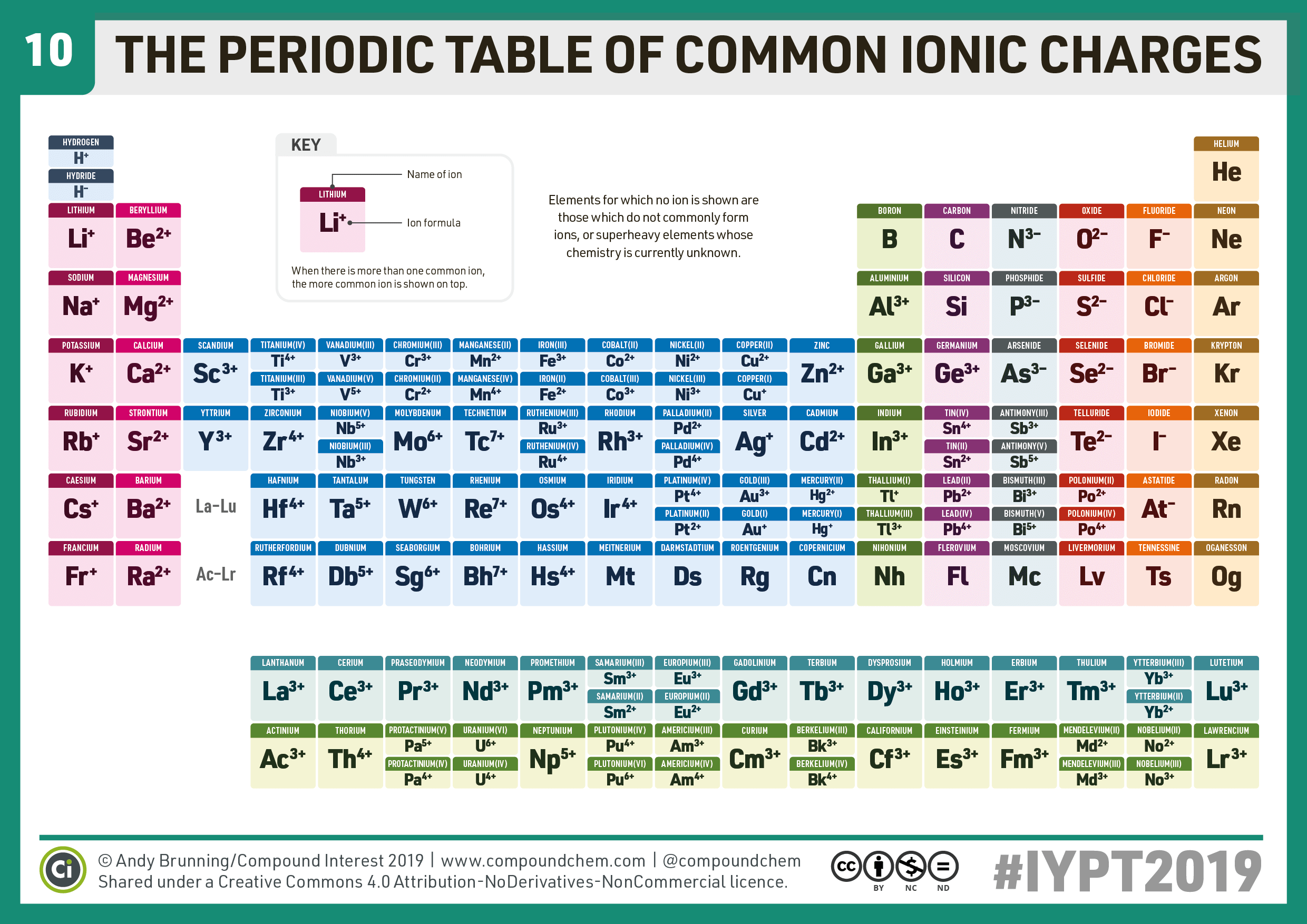
Ion Periodic Table Chart
Ion Periodic Table Chart - In chemistry, the definition of an ion is an electrically charged atom or molecule. Because an ion wants to become neutral by acquiring or. The meaning of ion is an atom or group of atoms that carries a positive or negative electric charge as a result of having lost or gained one or more electrons. An ion is an atom or molecule with either a positive or negative electrical charge. Atoms are made from positively charged protons, negatively charged. Ions are atoms or molecules that gain or lose electrons and carry an electrical charge. Positively charged ions are called cations; An ion is an atom or molecule with more or less electrons than usual, giving it a positive or negative electric charge. An atom or small group of atoms that has an electrical charge because it has added or lost one…. This means the atom or molecule has an unequal number of protons and electrons. It is charged so it will move near electricity. Ion an ion is an electrically charged atom or molecule (group of atoms). An atom or small group of atoms that has an electrical charge because it has added or lost one…. An ion is a charge carrying atom/molecule formed by the ionization process. An ion has a charge because the number of protons does not equal the number of electrons; This means the atom or molecule has an unequal number of protons and electrons. Ion, any atom or group of atoms that bears one or more positive or negative electrical charges. How to use ion in a. In chemistry, the definition of an ion is an electrically charged atom or molecule. An ion is an atom or molecule with either a positive or negative electrical charge. Ion an ion is an electrically charged atom or molecule (group of atoms). Ions are atoms or molecules that gain or lose electrons and carry an electrical charge. An ion is an atom or molecule with more or less electrons than usual, giving it a positive or negative electric charge. Ion, any atom or group of atoms that bears one. An ion is a charge carrying atom/molecule formed by the ionization process. How to use ion in a. An ion is an atom or molecule with either a positive or negative electrical charge. Ion an ion is an electrically charged atom or molecule (group of atoms). Cations are positively charged ions, while anions are negatively charged ions. Ion an ion is an electrically charged atom or molecule (group of atoms). The meaning of ion is an atom or group of atoms that carries a positive or negative electric charge as a result of having lost or gained one or more electrons. Cations are positively charged ions, while anions are negatively charged ions. The charge of an electron. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to. An atom or small group of atoms that has an electrical charge because it has added or lost one…. In chemistry, the definition of an ion is an electrically charged atom or molecule. Ion an ion is an electrically charged. The meaning of ion is an atom or group of atoms that carries a positive or negative electric charge as a result of having lost or gained one or more electrons. Positively charged ions are called cations; Ions are atoms or molecules that gain or lose electrons and carry an electrical charge. Cations are positively charged ions, while anions are. An ion is a charge carrying atom/molecule formed by the ionization process. Ions are atoms or molecules that gain or lose electrons and carry an electrical charge. An ion has a charge because the number of protons does not equal the number of electrons; The charge of an electron is considered to be negative by convention and this charge is. This means the atom or molecule has an unequal number of protons and electrons. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to. An ion is an atom or molecule with either a positive or negative electrical charge. The meaning of ion is an atom or group of atoms. Ion, any atom or group of atoms that bears one or more positive or negative electrical charges. An ion has a charge because the number of protons does not equal the number of electrons; The ratio of electrons and protons in an ionic species is never equal to 1. It is charged so it will move near electricity. An atom. Positively charged ions are called cations; How to use ion in a. Ions are atoms or molecules that gain or lose electrons and carry an electrical charge. The meaning of ion is an atom or group of atoms that carries a positive or negative electric charge as a result of having lost or gained one or more electrons. The ratio. An ion is an atom or molecule with either a positive or negative electrical charge. This means the atom or molecule has an unequal number of protons and electrons. Because an ion wants to become neutral by acquiring or. An ion is an atom or molecule with more or less electrons than usual, giving it a positive or negative electric. Ion an ion is an electrically charged atom or molecule (group of atoms). Ions are atoms or molecules that gain or lose electrons and carry an electrical charge. The ratio of electrons and protons in an ionic species is never equal to 1. This means the atom or molecule has an unequal number of protons and electrons. An ion has a charge because the number of protons does not equal the number of electrons; An ion is an atom or molecule with either a positive or negative electrical charge. An ion is a charge carrying atom/molecule formed by the ionization process. An atom or small group of atoms that has an electrical charge because it has added or lost one…. Ion, any atom or group of atoms that bears one or more positive or negative electrical charges. The meaning of ion is an atom or group of atoms that carries a positive or negative electric charge as a result of having lost or gained one or more electrons. Positively charged ions are called cations; The charge of an electron is considered to be negative by convention and this charge is equal and opposite to. Cations are positively charged ions, while anions are negatively charged ions. Because an ion wants to become neutral by acquiring or. An ion is an atom or molecule with more or less electrons than usual, giving it a positive or negative electric charge.Ion Chart For Elements
Periodic Table Of Ions 10 Free PDF Printables Printablee
Periodic Table With Ionic Charges
Periodic Table Of Ions Printable 2025 Periodic Table Printable
Periodic Table Of Ions 10 Free PDF Printables Printablee
Periodic Table Of Ions 10 Free PDF Printables Printablee
Periodic Table With Common Ionic Charges
Periodic Table Of Ions 10 Free PDF Printables Printablee
fessgrab Blog
Ion Chart For Elements
In Chemistry, The Definition Of An Ion Is An Electrically Charged Atom Or Molecule.
How To Use Ion In A.
Atoms Are Made From Positively Charged Protons, Negatively Charged.
It Is Charged So It Will Move Near Electricity.
Related Post:

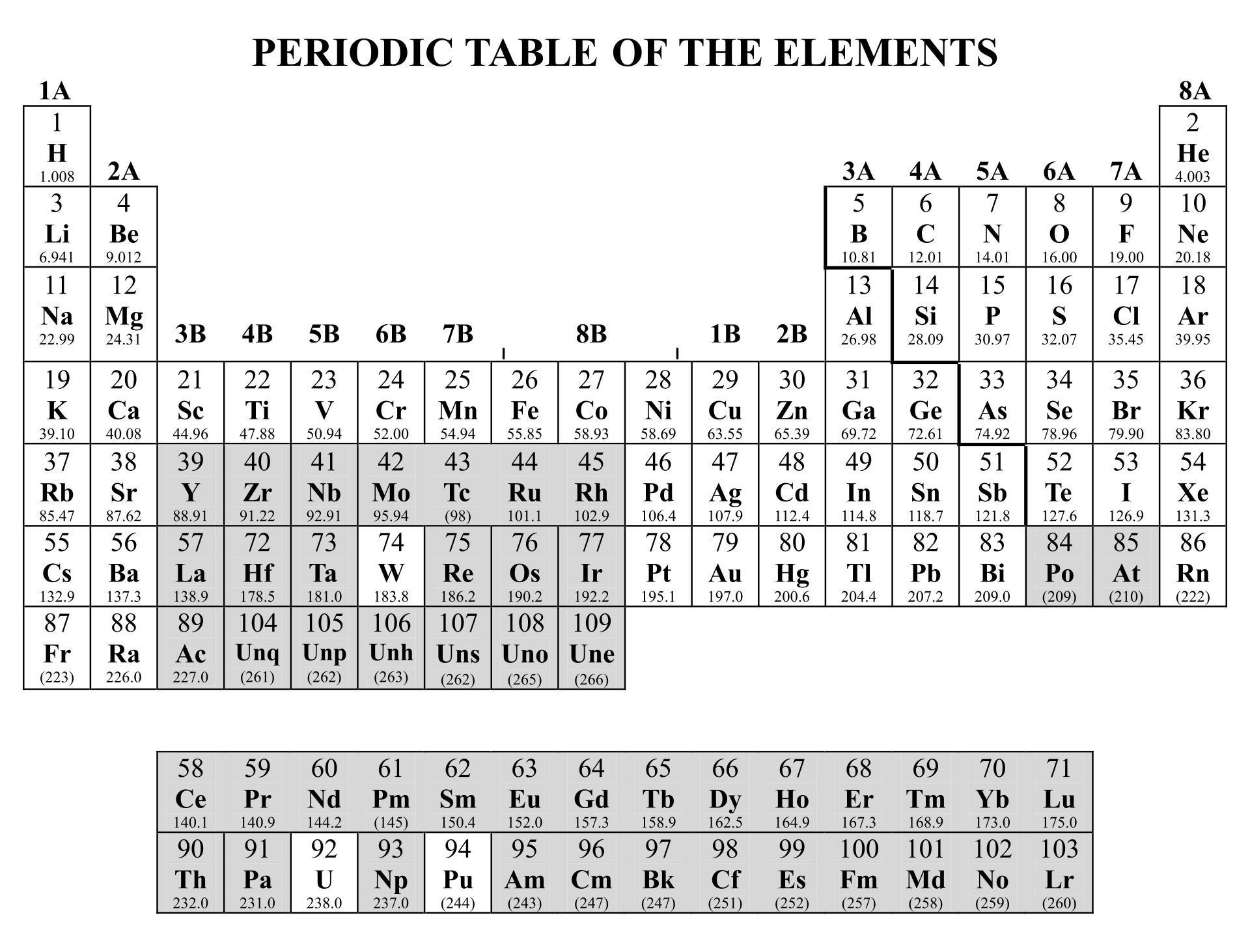


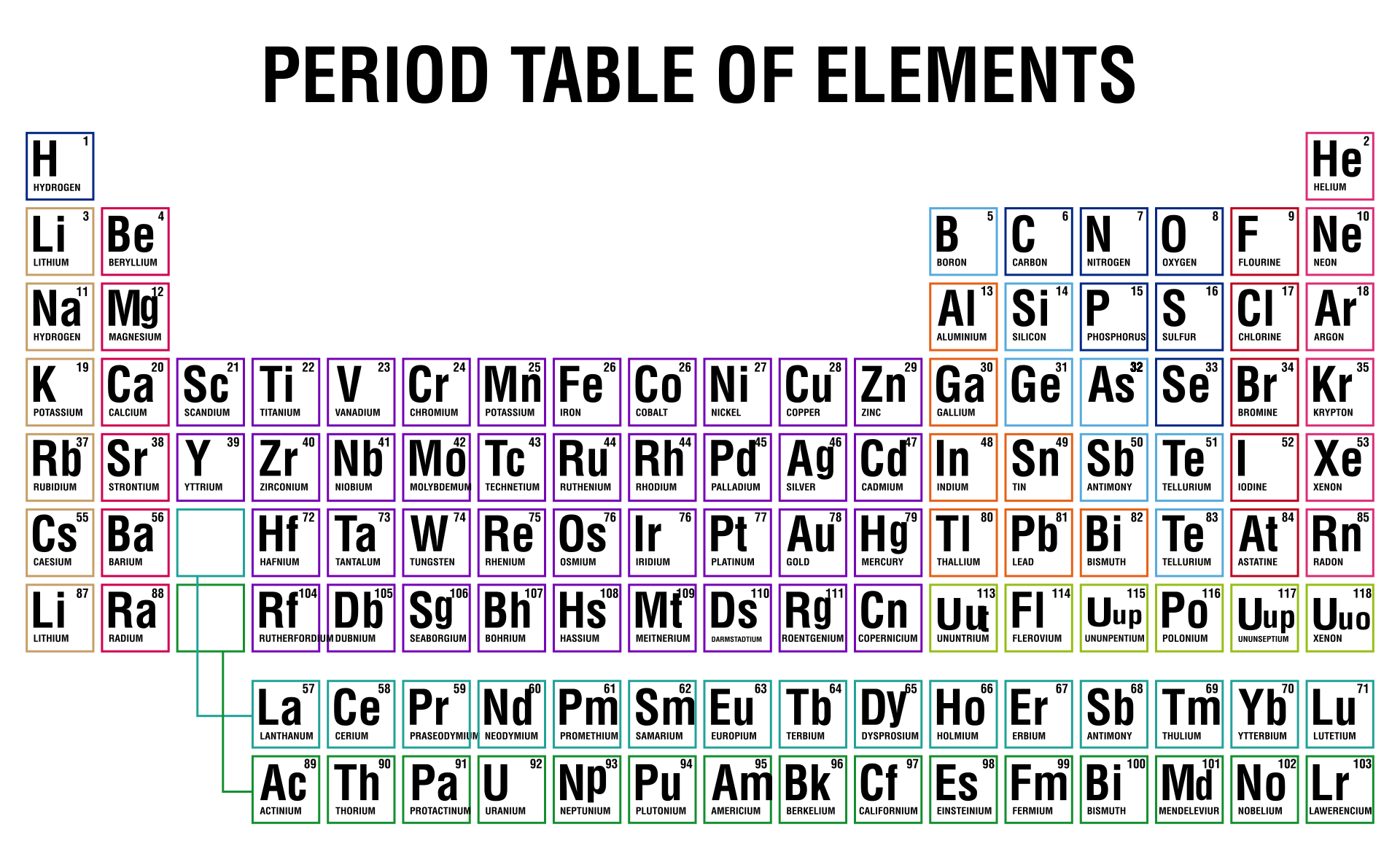
/PeriodicTableCharge-WBG-56a12db23df78cf772682c37.png)