Electron And Molecular Geometry Chart
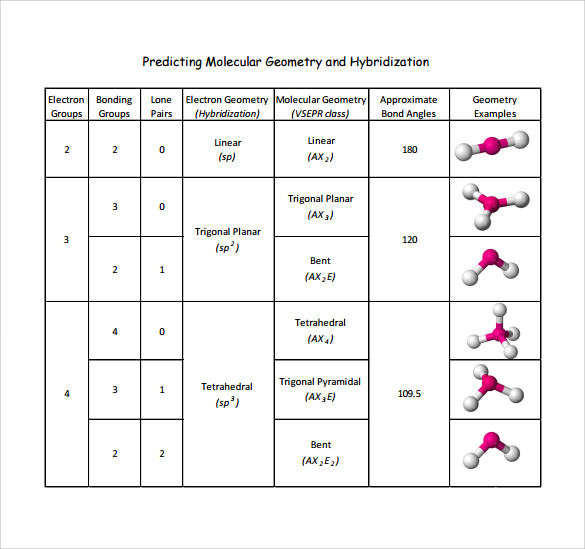
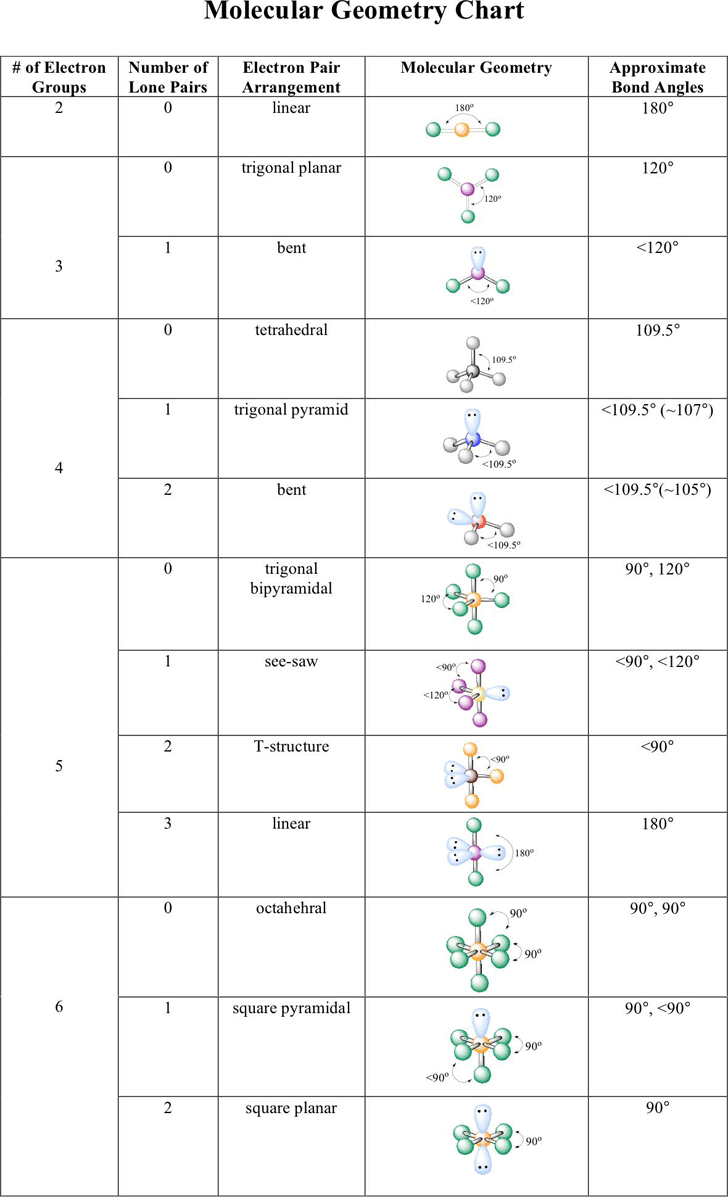
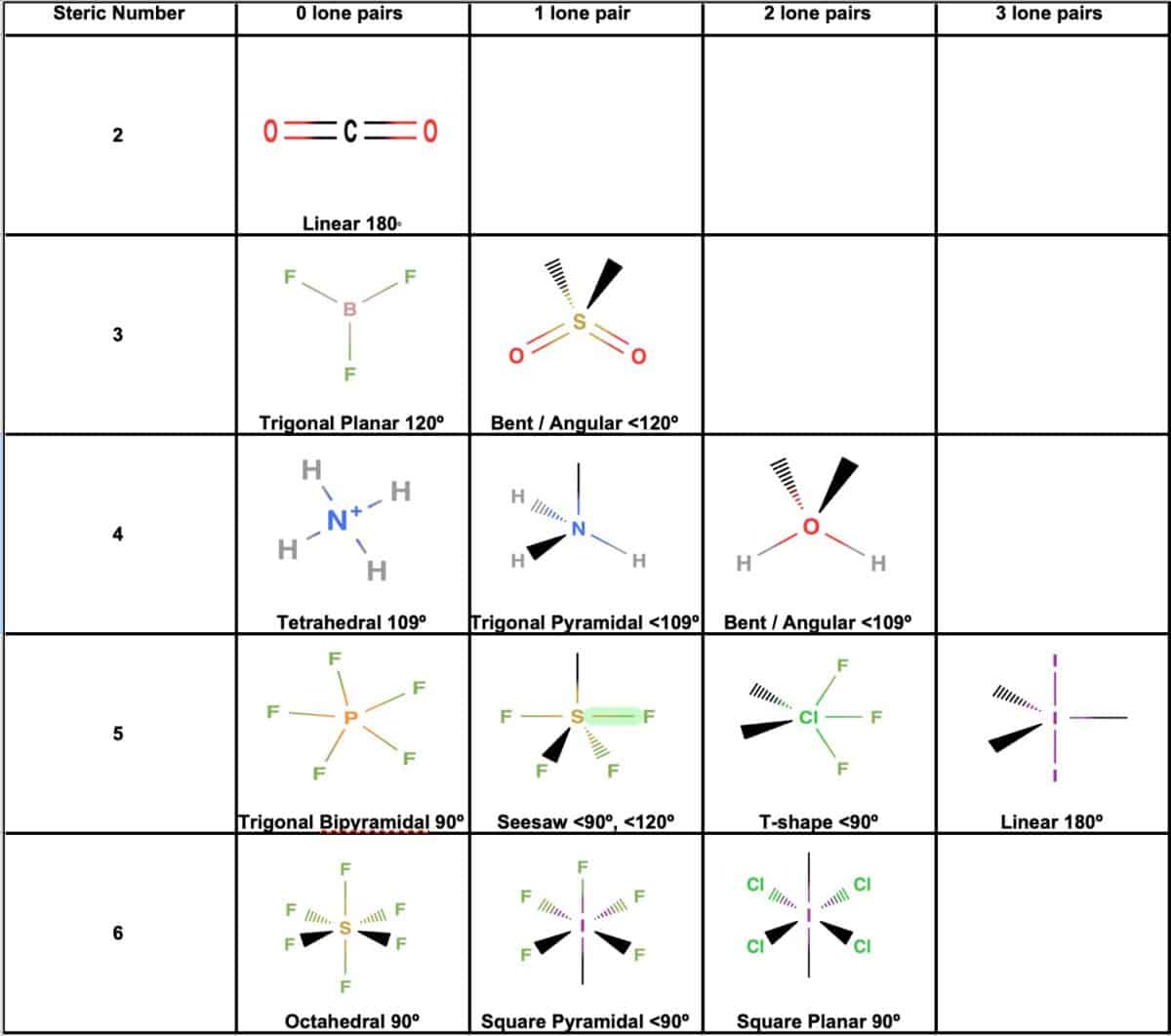
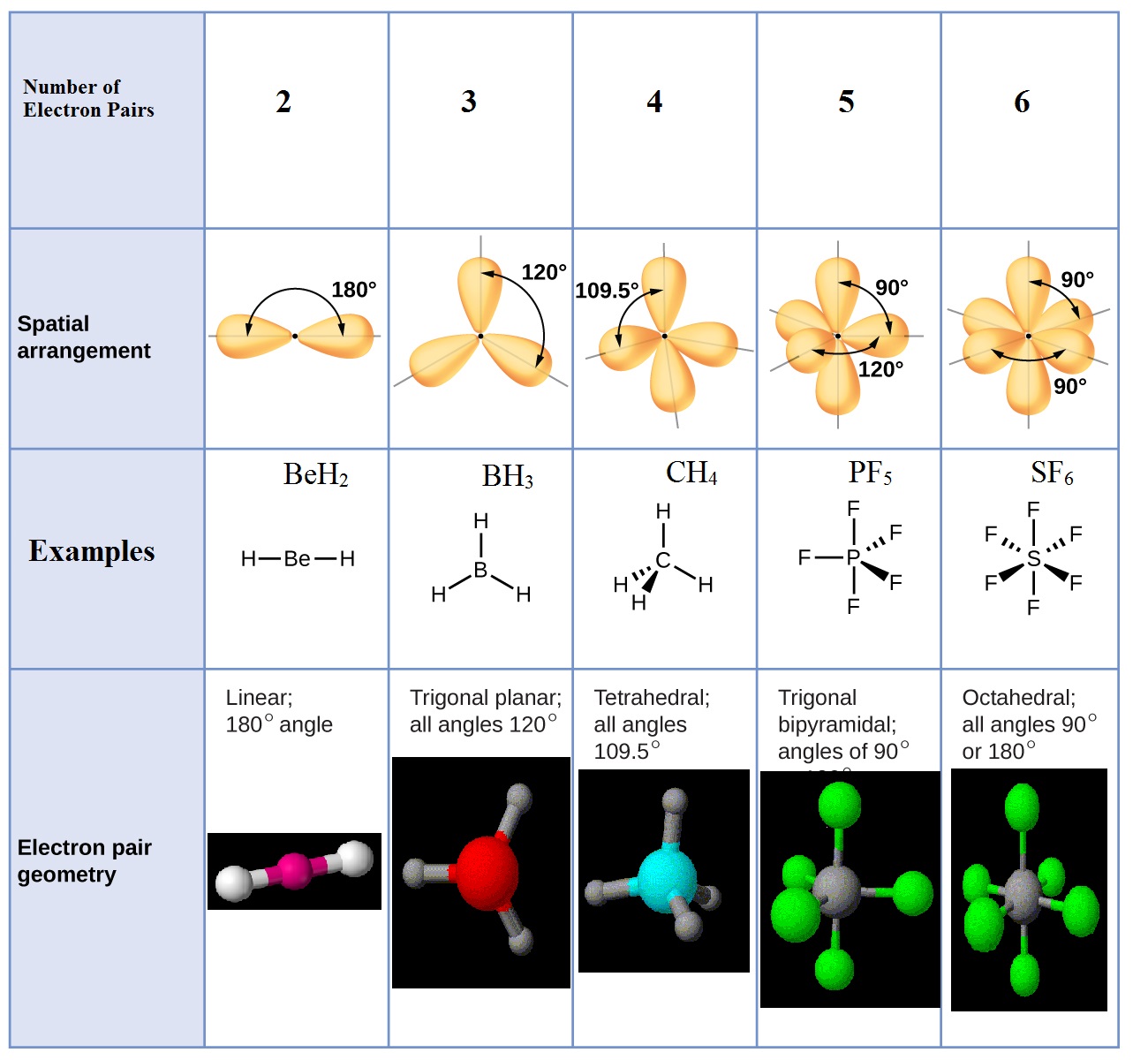
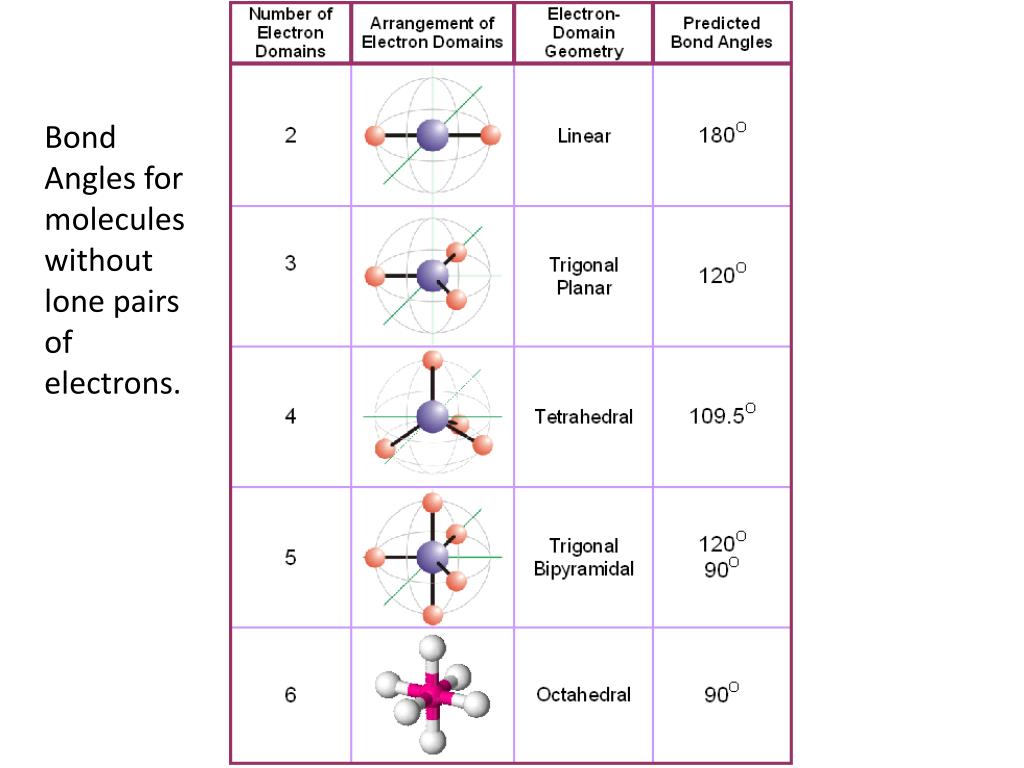
Electron And Molecular Geometry Chart - The molecular geometry differs from the electron geometry when a molecule contains one or more lone pairs. Join us as we define this subject, go over some examples, and list the different structures you will find in an electron and molecular geometry chart. The geometrical arrangements of the atoms are referred to as molecular geometry. You can manipulate the number of single, double, or triple bonds and lone pairs that are. Explore our table of common electron geometries with bonding domains, bond angles, and formulas. Check out a table of molecular geometries with examples and diagrams. This vsepr chart shows you all of the common vsepr geometries, organized by the steric number and how many lone electron pairs they have. We can use the vsepr model to predict the geometry of most polyatomic molecules and ions by focusing only on the number of electron pairs around the central atom, ignoring all other valence. You will need to know the electron pair geometries and molecular geometries. The steric number is how many atoms are bonded. The table below gives the electron pair geometries and molecular geometries for different molecules and ions. Check out a table of molecular geometries with examples and diagrams. If there are no lone pairs and all orbitals are bonding, then the molecular geometry is the electronic geometry. Join us as we define this subject, go over some examples, and list the different structures you will find in an electron and molecular geometry chart. We have also included some study guides to. You will need to know the electron pair geometries and molecular geometries. This vsepr chart shows you all of the common vsepr geometries, organized by the steric number and how many lone electron pairs they have. The geometrical arrangements of the atoms are referred to as molecular geometry. Lone pairs influence the molecular geometry, and so in this section we will look at. You can manipulate the number of single, double, or triple bonds and lone pairs that are. If there are no lone pairs and all orbitals are bonding, then the molecular geometry is the electronic geometry. We can use the vsepr model to predict the geometry of most polyatomic molecules and ions by focusing only on the number of electron pairs around the central atom, ignoring all other valence. What are the different shapes of molecules. We. We can use the vsepr model to predict the geometry of most polyatomic molecules and ions by focusing only on the number of electron pairs around the central atom, ignoring all other valence. The steric number is how many atoms are bonded. The molecular geometry differs from the electron geometry when a molecule contains one or more lone pairs. Check. We have also included some study guides to. This vsepr chart shows you all of the common vsepr geometries, organized by the steric number and how many lone electron pairs they have. Join us as we define this subject, go over some examples, and list the different structures you will find in an electron and molecular geometry chart. You will. If there are no lone pairs and all orbitals are bonding, then the molecular geometry is the electronic geometry. You will need to know the electron pair geometries and molecular geometries. Join us as we define this subject, go over some examples, and list the different structures you will find in an electron and molecular geometry chart. The molecular geometry. What are the different shapes of molecules. The geometrical arrangements of the atoms are referred to as molecular geometry. Explore our table of common electron geometries with bonding domains, bond angles, and formulas. You will need to know the electron pair geometries and molecular geometries. You can manipulate the number of single, double, or triple bonds and lone pairs that. Lone pairs influence the molecular geometry, and so in this section we will look at. You can manipulate the number of single, double, or triple bonds and lone pairs that are. If there are no lone pairs and all orbitals are bonding, then the molecular geometry is the electronic geometry. The molecular geometry differs from the electron geometry when a. If there are no lone pairs and all orbitals are bonding, then the molecular geometry is the electronic geometry. The molecular geometry differs from the electron geometry when a molecule contains one or more lone pairs. Check out a table of molecular geometries with examples and diagrams. You will need to know the electron pair geometries and molecular geometries. You. Lone pairs influence the molecular geometry, and so in this section we will look at. This vsepr chart shows you all of the common vsepr geometries, organized by the steric number and how many lone electron pairs they have. We have also included some study guides to. The steric number is how many atoms are bonded. What are the different. If there are no lone pairs and all orbitals are bonding, then the molecular geometry is the electronic geometry. The table below gives the electron pair geometries and molecular geometries for different molecules and ions. The steric number is how many atoms are bonded. This vsepr chart shows you all of the common vsepr geometries, organized by the steric number. The geometrical arrangements of the atoms are referred to as molecular geometry. Check out a table of molecular geometries with examples and diagrams. The table below gives the electron pair geometries and molecular geometries for different molecules and ions. The molecular geometry differs from the electron geometry when a molecule contains one or more lone pairs. You will need to. The steric number is how many atoms are bonded. Join us as we define this subject, go over some examples, and list the different structures you will find in an electron and molecular geometry chart. You can manipulate the number of single, double, or triple bonds and lone pairs that are. Check out a table of molecular geometries with examples and diagrams. Explore our table of common electron geometries with bonding domains, bond angles, and formulas. What are the different shapes of molecules. The molecular geometry differs from the electron geometry when a molecule contains one or more lone pairs. Lone pairs influence the molecular geometry, and so in this section we will look at. The geometrical arrangements of the atoms are referred to as molecular geometry. The table below gives the electron pair geometries and molecular geometries for different molecules and ions. If there are no lone pairs and all orbitals are bonding, then the molecular geometry is the electronic geometry. This vsepr chart shows you all of the common vsepr geometries, organized by the steric number and how many lone electron pairs they have.Electron geometry chart and examples numbersaad
Electron Domain and Molecular Geometry Chart
electrongeometrychartmoleculargeometrychartexample1 electron geometry chart Molecular
FREE 8+ Sample Molecular Geometry Chart Templates in PDF MS Word
Free Molecular Geometry Chart PDF 603KB 1 Page(s)
Molecular Geometry Boundless Chemistry
Electron Geometry Chart Chemistry molecular geometry electron geometries chart shapes vsepr
Electron Geometry Chart Chemistry molecular geometry electron geometries chart shapes vsepr
Electron pair geometry and molecular geometry chart volfplus
Electron and molecular geometry chart examples heryhd
We Can Use The Vsepr Model To Predict The Geometry Of Most Polyatomic Molecules And Ions By Focusing Only On The Number Of Electron Pairs Around The Central Atom, Ignoring All Other Valence.
We Have Also Included Some Study Guides To.
You Will Need To Know The Electron Pair Geometries And Molecular Geometries.
Related Post: