Dipole Length Chart
Dipole Length Chart - This is because the charges will. The dipole field at a point located at → r away from the origin set at the center of a dipole is: Dipoles are created when one element has a higher electronegativity (tendency to attract electrons) than another in a bond. When the length of carbon chain increases the van der waals dispersion forces increase which results in an increase in the strength and size of the temporarily induced dipole. Donate or accept protons with itself) dichloromethane and acetone are good. → e = → e 1 + → e 2 → e = kq→ r 1 r3 1 − kq→ r 2 r3 2 given q and d, if you know. What type of intermolecular force would water. Which intermolecular forces in h2o make ice less dense than liquid water: These dipoles may be permanent (polar molecules) or. It’s net dipole moment is not zero. Dipoles are created when one element has a higher electronegativity (tendency to attract electrons) than another in a bond. These dipoles may be permanent (polar molecules) or. This is because the charges will. Which intermolecular forces in h2o make ice less dense than liquid water: Solvents that contain a strong dipole moment but don't participate in hydrogen bonding (ie. Donate or accept protons with itself) dichloromethane and acetone are good. The dipole moment of pcl_3 is greater. It’s net dipole moment is not zero. The electronegativity difference has to. > bb (ph_3) the electron geometry of ph_3 is tetrahedral and the molecular geometry is trigonal pyramidal. What type of intermolecular force would water. For an electric dipole to exist an external electric field is required. When the length of carbon chain increases the van der waals dispersion forces increase which results in an increase in the strength and size of the temporarily induced dipole. Dipoles are created when one element has a higher electronegativity (tendency to. Dipoles are created when one element has a higher electronegativity (tendency to attract electrons) than another in a bond. What type of intermolecular force would water. The electronegativity difference has to. When the length of carbon chain increases the van der waals dispersion forces increase which results in an increase in the strength and size of the temporarily induced dipole.. Donate or accept protons with itself) dichloromethane and acetone are good. Dipoles are created when one element has a higher electronegativity (tendency to attract electrons) than another in a bond. This is because the charges will. Van der waals forces are considered to be intermolecular forces, along with hydrogen bonds dipole dipole forces, these forces tend to bond or attract. The dipole field at a point located at → r away from the origin set at the center of a dipole is: It’s net dipole moment is not zero. Two charges alone (one positive and one negative) will not be stable. Solvents that contain a strong dipole moment but don't participate in hydrogen bonding (ie. > bb (ph_3) the electron. Two charges alone (one positive and one negative) will not be stable. Dipoles are created when one element has a higher electronegativity (tendency to attract electrons) than another in a bond. Is ch3co2h a dipole dipole? Van der waals forces are considered to be intermolecular forces, along with hydrogen bonds dipole dipole forces, these forces tend to bond or attract. Which intermolecular forces in h2o make ice less dense than liquid water: What type of intermolecular force would water. The electronegativity difference has to. For an electric dipole to exist an external electric field is required. It’s net dipole moment is not zero. The electronegativity difference has to. > bb (ph_3) the electron geometry of ph_3 is tetrahedral and the molecular geometry is trigonal pyramidal. What type of intermolecular force would water. The dipole field at a point located at → r away from the origin set at the center of a dipole is: Solvents that contain a strong dipole moment but don't. > bb (ph_3) the electron geometry of ph_3 is tetrahedral and the molecular geometry is trigonal pyramidal. Donate or accept protons with itself) dichloromethane and acetone are good. The dipole field at a point located at → r away from the origin set at the center of a dipole is: It’s net dipole moment is not zero. Solvents that contain. It’s net dipole moment is not zero. The dipole field at a point located at → r away from the origin set at the center of a dipole is: Which intermolecular forces in h2o make ice less dense than liquid water: The electronegativity difference has to. Van der waals forces are considered to be intermolecular forces, along with hydrogen bonds. The electronegativity difference has to. It’s net dipole moment is not zero. Van der waals forces are considered to be intermolecular forces, along with hydrogen bonds dipole dipole forces, these forces tend to bond or attract molecules to molecules. When the length of carbon chain increases the van der waals dispersion forces increase which results in an increase in the. Is ch3co2h a dipole dipole? This is because the charges will. Solvents that contain a strong dipole moment but don't participate in hydrogen bonding (ie. → e = → e 1 + → e 2 → e = kq→ r 1 r3 1 − kq→ r 2 r3 2 given q and d, if you know. These dipoles may be permanent (polar molecules) or. It’s net dipole moment is not zero. Donate or accept protons with itself) dichloromethane and acetone are good. The dipole moment of pcl_3 is greater. Two charges alone (one positive and one negative) will not be stable. Dipoles are created when one element has a higher electronegativity (tendency to attract electrons) than another in a bond. Which intermolecular forces in h2o make ice less dense than liquid water: The dipole field at a point located at → r away from the origin set at the center of a dipole is: The electronegativity difference has to. Van der waals forces are considered to be intermolecular forces, along with hydrogen bonds dipole dipole forces, these forces tend to bond or attract molecules to molecules.Comparative chart for straight and Vdipole antennas. Download Table
Dipole Length Chart Ponasa
Ham Radio Dipole Antenna Calculator at Edward Calvo blog
Dipole Length Chart Ponasa
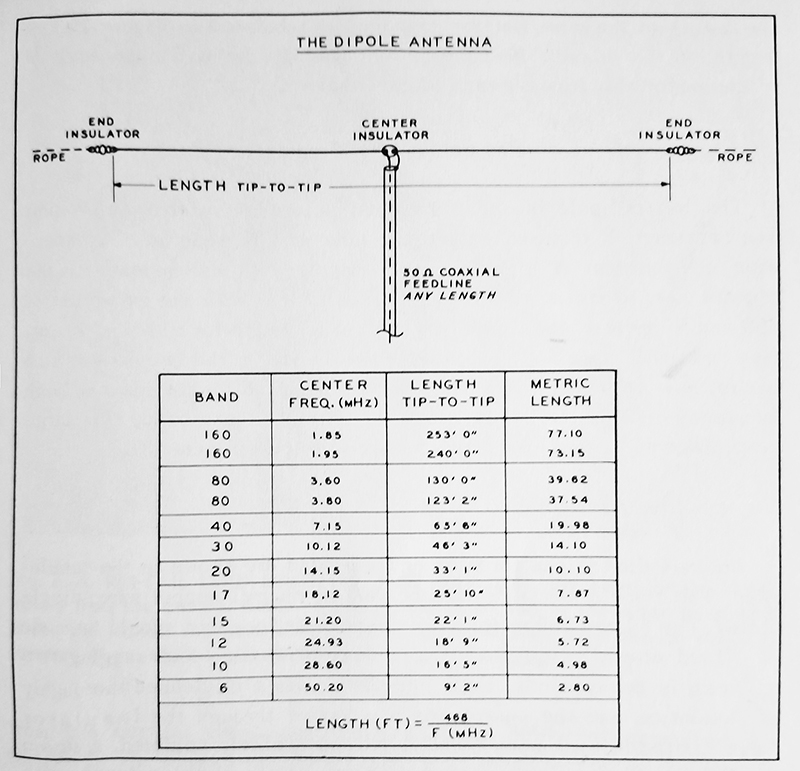
Dipole Antenna Length Calculator
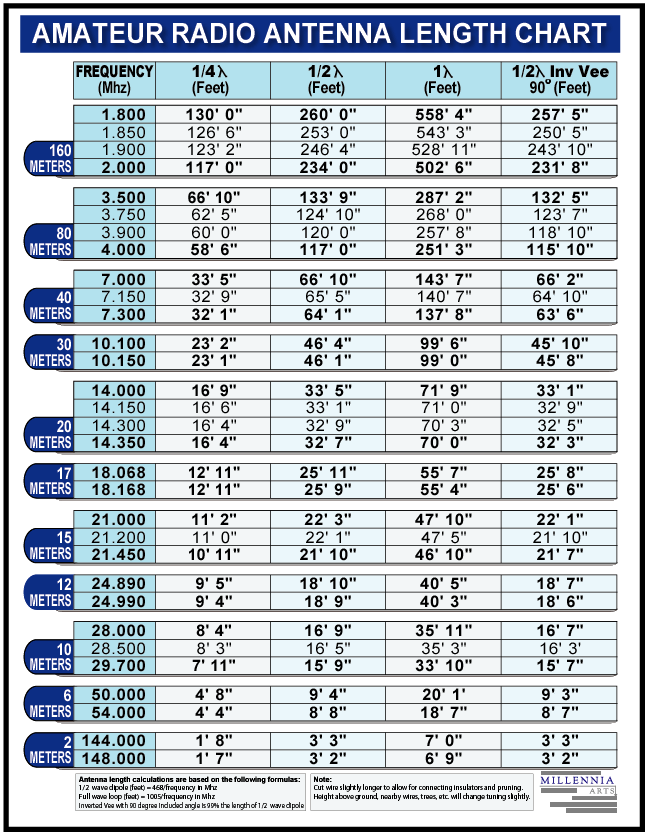
Dipole Antenna Length Chart
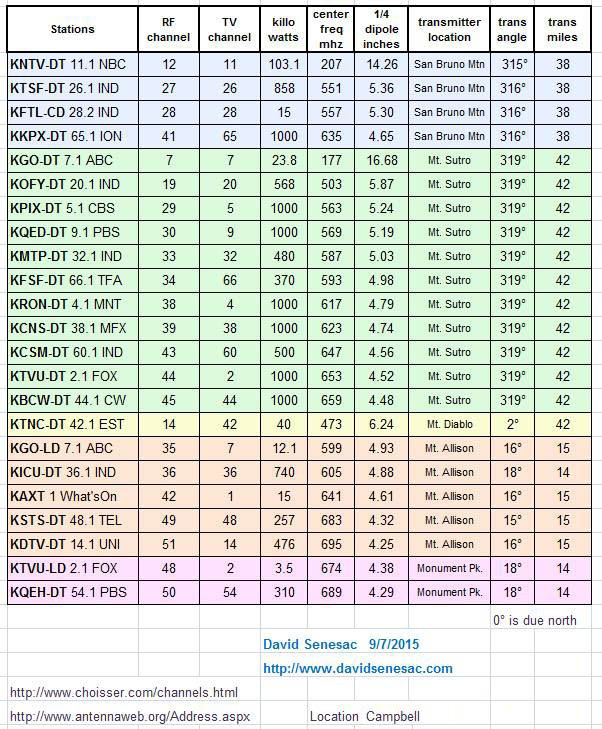
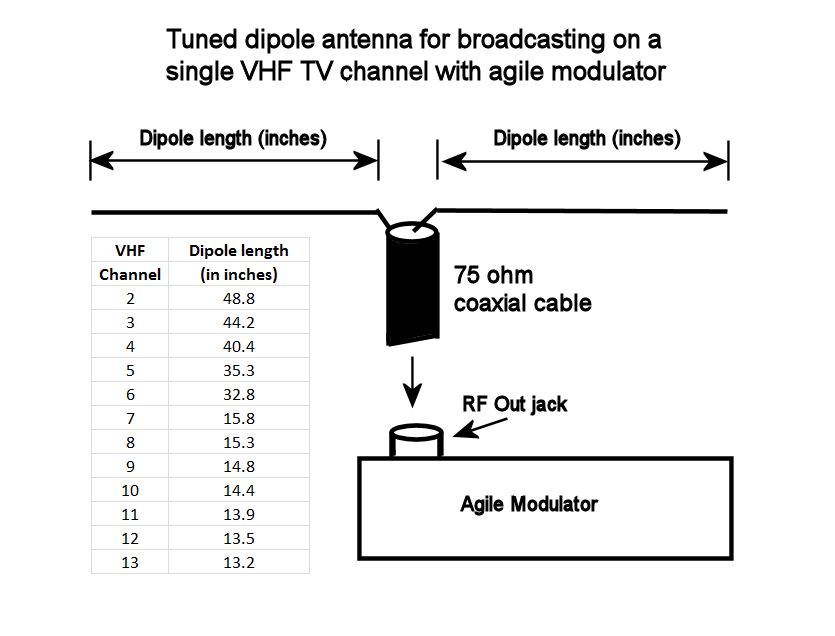
Creating a Home TV Transmitter
Dipole Length Chart Portal.posgradount.edu.pe
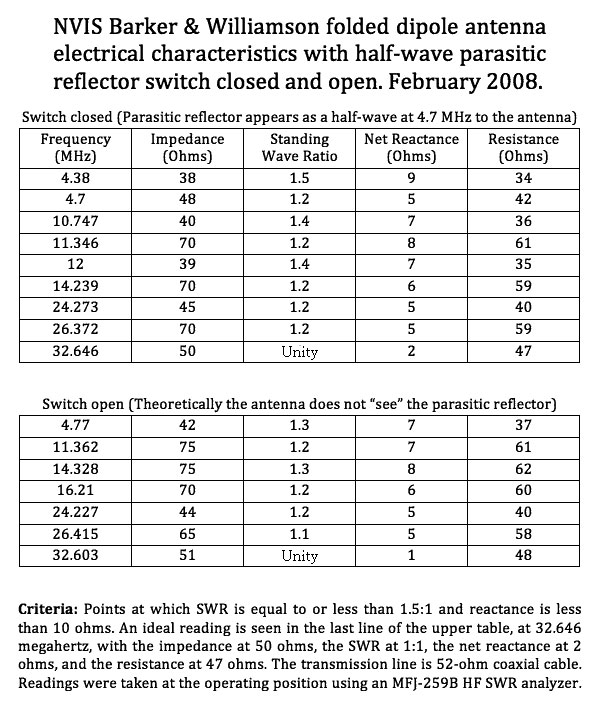
VHF Dipole Antenna Length Calculator
dipole antenna length chart Coaxial folded dipole antenna
For An Electric Dipole To Exist An External Electric Field Is Required.
What Type Of Intermolecular Force Would Water.
When The Length Of Carbon Chain Increases The Van Der Waals Dispersion Forces Increase Which Results In An Increase In The Strength And Size Of The Temporarily Induced Dipole.
> Bb (Ph_3) The Electron Geometry Of Ph_3 Is Tetrahedral And The Molecular Geometry Is Trigonal Pyramidal.
Related Post: