Basicity Chart
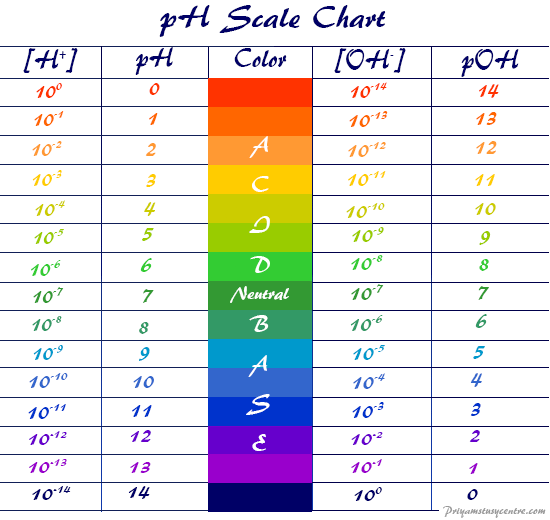
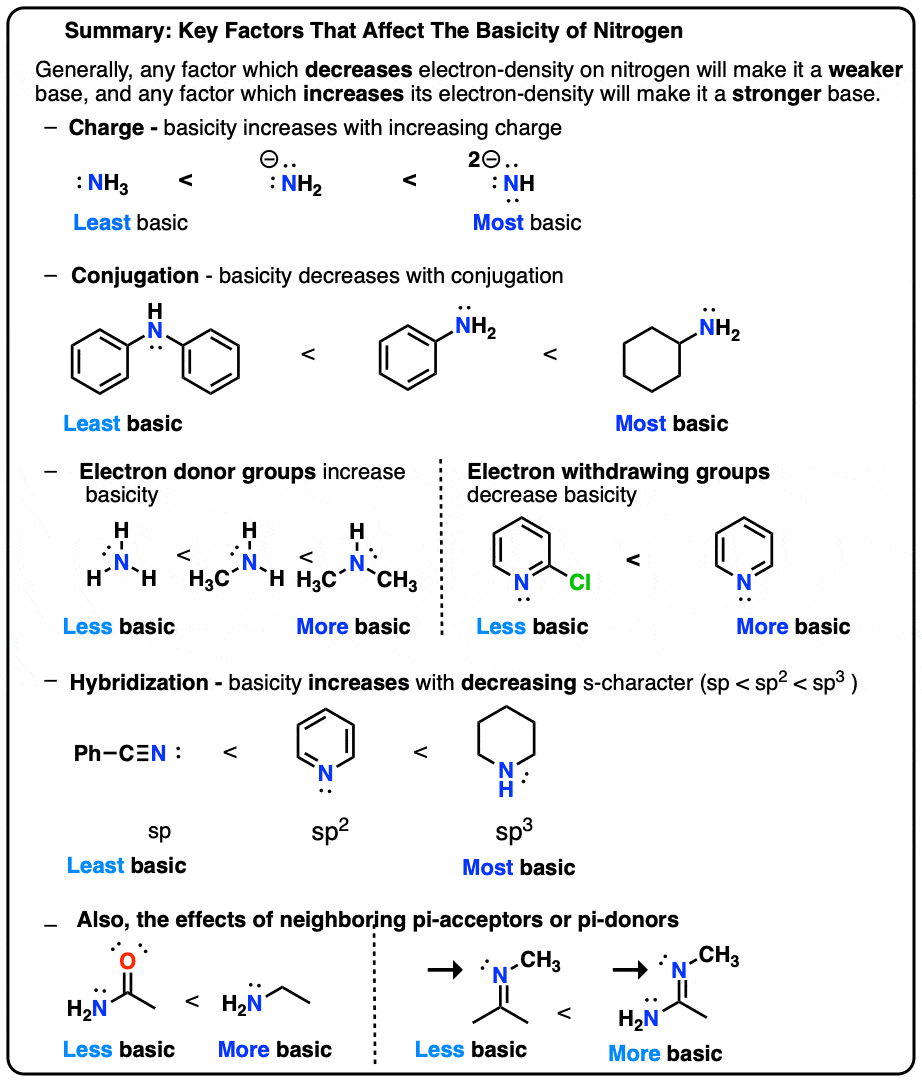
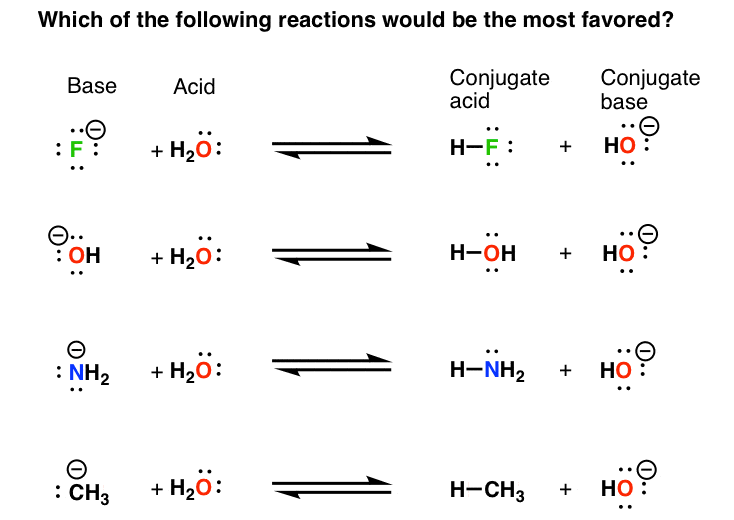
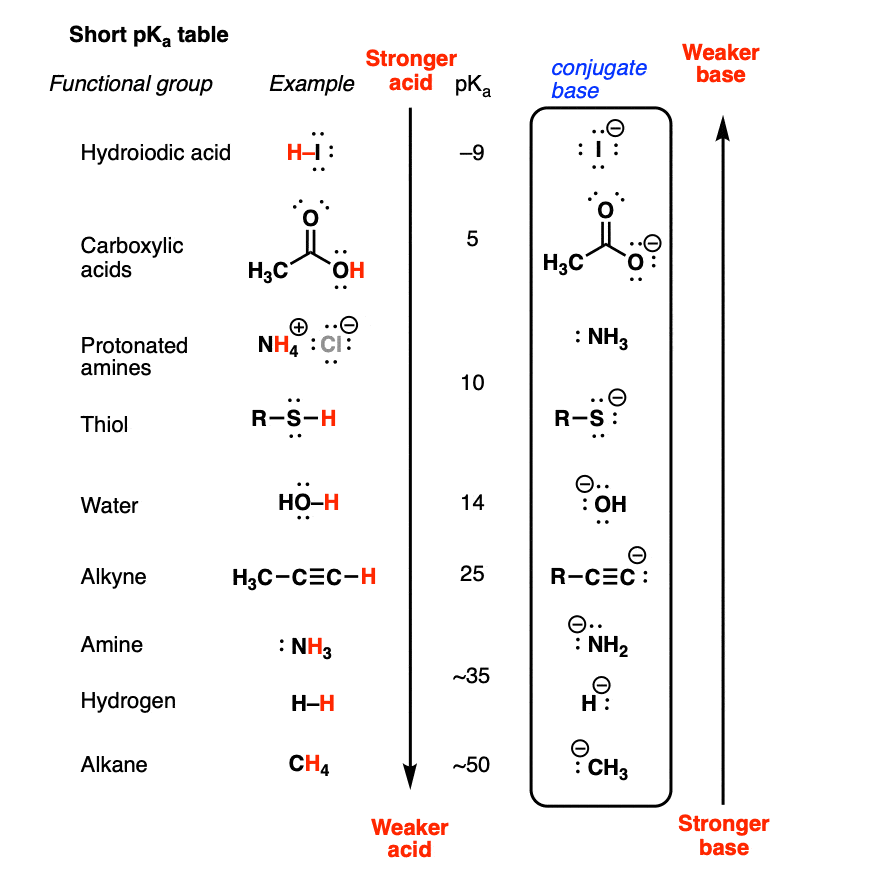
Basicity Chart - Now i have a problem with the first line. The issue is that basicity has a couple of meanings and you have to be able to tell from the context what it means. I know that to compare the basic strengths, we need to find the stability of their. I need to find the decreasing basicity of the following compounds(i, ii, iii, iv, respectively): Order of basicity for arylamines and ammonia in gas phase ask question asked 3 years, 8 months ago modified 3 years, 5 months ago Greater is the stability of the substituted. Thus, the order of basicity of aliphatic amines should be: And that bihx3 b i h x 3 should have. The notable drop is due to the difference of the. The first is logical, and is used to describe how basic a base is. Now i have a problem with the first line. I know that to compare the basic strengths, we need to find the stability of their. Now i end up thinking basicity and reducing character are the same thing. The notable drop is due to the difference of the. My book defines the basicity of the acid as the number of hx+ h x + ions furnished by one mole of the acid in solution. The first is logical, and is used to describe how basic a base is. I need to find the decreasing basicity of the following compounds(i, ii, iii, iv, respectively): While steric hindrance will decrease the rate solvent access, it more importantly also. Well, i know that b(oh)x3 b (o h) x 3 is a weak. Basicity decreases with increasing s character basicity decreases with increasing stability, due to resonance/ delocalization (taken from this webpage). The issue is that basicity has a couple of meanings and you have to be able to tell from the context what it means. Order of basicity for arylamines and ammonia in gas phase ask question asked 3 years, 8 months ago modified 3 years, 5 months ago Greater is the stability of the substituted. And that bihx3 b i. Thus, the order of basicity of aliphatic amines should be: Basicity is how easily can the compound give up hydrogen atoms/electrons. And that bihx3 b i h x 3 should have. The notable drop is due to the difference of the. Order of basicity for arylamines and ammonia in gas phase ask question asked 3 years, 8 months ago modified. The order of acidity and basicity is based on countless experiments that establish. Now i end up thinking basicity and reducing character are the same thing. Basicity is how easily can the compound give up hydrogen atoms/electrons. Acidity and basicity are experimentally determined. The first is logical, and is used to describe how basic a base is. Acidity and basicity depend on the interaction between the acid/base and the solvent. Basicity is how easily can the compound give up hydrogen atoms/electrons. My book defines the basicity of the acid as the number of hx+ h x + ions furnished by one mole of the acid in solution. Order of basicity for arylamines and ammonia in gas phase. The issue is that basicity has a couple of meanings and you have to be able to tell from the context what it means. The order of acidity and basicity is based on countless experiments that establish. I know that to compare the basic strengths, we need to find the stability of their. I need to find the decreasing basicity. I need to find the decreasing basicity of the following compounds(i, ii, iii, iv, respectively): My book defines the basicity of the acid as the number of hx+ h x + ions furnished by one mole of the acid in solution. Now i end up thinking basicity and reducing character are the same thing. Now i have a problem with. The issue is that basicity has a couple of meanings and you have to be able to tell from the context what it means. My book defines the basicity of the acid as the number of hx+ h x + ions furnished by one mole of the acid in solution. Now i end up thinking basicity and reducing character are. Order of basicity for arylamines and ammonia in gas phase ask question asked 3 years, 8 months ago modified 3 years, 5 months ago The first is logical, and is used to describe how basic a base is. I need to find the decreasing basicity of the following compounds(i, ii, iii, iv, respectively): Greater is the stability of the substituted.. Thus, the order of basicity of aliphatic amines should be: Order of basicity for arylamines and ammonia in gas phase ask question asked 3 years, 8 months ago modified 3 years, 5 months ago Now i end up thinking basicity and reducing character are the same thing. I know that to compare the basic strengths, we need to find the. I need to find the decreasing basicity of the following compounds(i, ii, iii, iv, respectively): Basicity is how easily can the compound give up hydrogen atoms/electrons. My book defines the basicity of the acid as the number of hx+ h x + ions furnished by one mole of the acid in solution. Order of basicity for arylamines and ammonia in. While steric hindrance will decrease the rate solvent access, it more importantly also. Greater is the stability of the substituted. Thus, the order of basicity of aliphatic amines should be: The order of acidity and basicity is based on countless experiments that establish. 6 realise that there is a notable drop in basicity from nitrogen to phosphorus and then a slow and continuous further diminishing. Now i have a problem with the first line. I need to find the decreasing basicity of the following compounds(i, ii, iii, iv, respectively): Acidity and basicity are experimentally determined. Well, i know that b(oh)x3 b (o h) x 3 is a weak. I know that to compare the basic strengths, we need to find the stability of their. And that bihx3 b i h x 3 should have. The first is logical, and is used to describe how basic a base is. Basicity decreases with increasing s character basicity decreases with increasing stability, due to resonance/ delocalization (taken from this webpage). My book defines the basicity of the acid as the number of hx+ h x + ions furnished by one mole of the acid in solution. The notable drop is due to the difference of the. Acidity and basicity depend on the interaction between the acid/base and the solvent.pH Scale pOH Scale Definition, Range, Chart, Measurement
Acidity and Basicity Constants Acidity and basicity consta… Flickr Photo Sharing!
Secondary Amines Examples at David Masterson blog
Understanding basicity in organic chemistry — Master Organic Chemistry
Acids and Bases
Acidity, Basicity, and Stability The Relationship YouTube
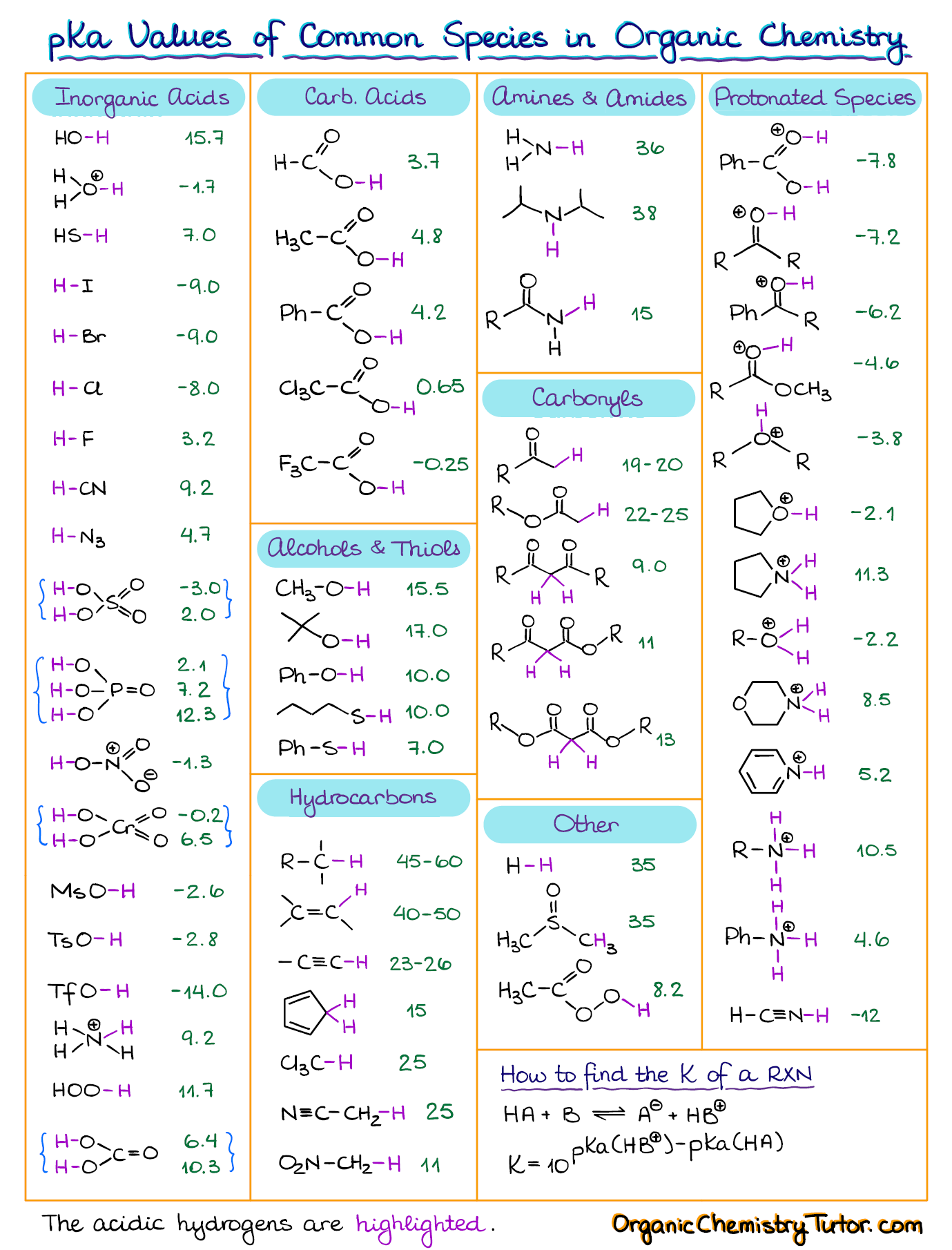
AcidBase Equilibrium Part 1 How to Use the pKa Table — Organic Chemistry Tutor
Acids And Bases List Strength
Order of Basicity Acid and Base Strengths YouTube
Ionization Constants of BH Onium Acids Name of Base (B) Formula of B K (BH ) pK
Basicity Is How Easily Can The Compound Give Up Hydrogen Atoms/Electrons.
Order Of Basicity For Arylamines And Ammonia In Gas Phase Ask Question Asked 3 Years, 8 Months Ago Modified 3 Years, 5 Months Ago
Primary > Secondary > Tertiary.
Now I End Up Thinking Basicity And Reducing Character Are The Same Thing.
Related Post: